{wmv}carotid stenting ch10{/wmv}
{mp4-flv}acces carotide{/mp4-flv}







Angioplasty and Stenting of the Extracranial Carotid Arteries
Michel Henry, MD, Max Amor, MD, Christos Klonaris, MD, Isabelle Henry, MD, Isabelle Masson, MD, Zukai Chati, MD, Edmond Leborgne, MD, and Michèle Hugel, RN
The Department of Interventional Cardiology, U.C.C.I. Polyclinique d’Essey, 54270 Essey-les-Nancy, France (Henry) (Amor) (Klonaris) (Henry) (Masson) (Chati) (Leborgne) (Hugel)
Zvonimir Krajcer, MD, Section Editor
This article has been cited by other articles in PMC.
Abstract
We performed percutaneous transluminal angioplasty and stenting in patients with carotid artery stenosis to determine the efficacy of these techniques as an alternative to surgical endarterectomy.
From April 1995 through July 1999, 315 carotid angioplasty procedures were performed (right, 151; left, 164) in 290 patients ranging in age from 40 to 93 years. Of these patients, 42% were symptomatic and 58% were asymptomatic. Twenty-five patients underwent bilateral procedures. The mean percentage of stenosis was 82.3% ± 8.7% SD. Angioplasty and stenting were performed without cerebral protection in 165 arteries and with protection in 150. Two methods of protection were used: the Theron technique and the PercuSurge® Guardwire™ temporary occlusion and aspiration system.
Balloon dilation and stent placement were successful in 289 patients; in the last patient, severe arterial tortuosity prevented catheterization and stenting. We observed 13 periprocedural neurologic complications due to ischemia (4.2%): 4 transient ischemic attacks (1.3%), 4 minor strokes (1.3%), and 5 major strokes (1.6%), including 1 death. At 6 months, 210 patients had a follow-up angiogram (155) or duplex ultrasound (55). There were 10 restenoses (4.7%), 1 of which was symptomatic and 2 of which showed mild compression of a Palmaz stent without marked stenosis. Primary and secondary 4-year patency rates were 96% and 99%, respectively.
These results demonstrate acceptable mortality and morbidity rates related to carotid angioplasty and stenting. However, we found the risk of embolic stroke to be substantial. Cerebral protection may improve the results of carotid angioplasty and expand the indications for this procedure.
Key words: Angioplasty, transluminal percutaneous; arterial occlusive diseases; carotid artery diseases; carotid stenosis/surgery; carotid stenosis/therapy; cerebral ischemia; embolism; stents; vascular patency
Cerebrovascular accidents remain a major public health problem. They are the 3rd leading cause of death in the world, accounting for 500,000 strokes per year in the United States and 150,000 in France. One third of patients who sustain an ischemic stroke die, and another third are permanently disabled. 1 Carotid artery stenosis is the cause in 20% to 30% of all cases. 2 The natural history of the disease is directly related to the severity of the lesion. 3 Patients with a stenosis greater than 75% have a 2% to 5% risk of suffering an ischemic stroke during the 1st year. 4,5 After a transient ischemic attack (TIA), the risk of subsequent stroke is 12% to 13% during the 1st year and 30% to 37% within 5 years. 6,7 A program of prevention and treatment of carotid artery stenosis is therefore necessary.
Prospective randomized studies, such as the North American Symptomatic Carotid Endarterectomy Trial (NASCET), the European Carotid Surgery Trial (ECST), and the Asymptomatic Carotid Atherosclerosis Study (ACAS), 8–12 proved the superiority of carotid endarterectomy over medical treatment for both symptomatic and asymptomatic patients with severe carotid stenosis. However, the inherent risks of surgery must be considered. The risk of stroke was 5.8% in the NASCET, 9 7.5% in the ECST, 11 and 2.3% in the ACAS. 12 Rothwell and colleagues 13 reviewed 50 surgical series and found the detection of cerebrovascular accidents to be as high as 7.7% when a postprocedural follow-up was performed by neurologists. Patients with associated coronary artery disease have up to 18% mortality and morbidity rates after carotid endarterectomy. 8,14,15 Other complications of carotid endar-terectomy include cranial nerve palsies (7.6% to 27%), 8–17 hematomas (5.5%), 8 and restenoses (5% to 9%). 18,19
Angioplasty has proved its efficacy in coronary and peripheral arteries and, therefore, may be a reasonable alternative to surgery for carotid artery stenosis. Endovascular techniques are still not widely accepted because of the risk of cerebral embolism, but the use of cerebral protection devices may limit that risk in the future.
We report our experience with carotid artery angioplasty and stenting in 290 patients.
Patients and Methods
From April 1995 through July 1999, we performed 315 carotid angioplasty procedures (right, 151; left, 164) in 290 patients (213 men and 77 women). The mean age was 70.5 ± 9.5 SD years (range, 40 to 93 years). Of these patients, 42% were symptomatic and 58% were asymptomatic. Twenty-five patients underwent bilateral interventions as staged procedures during the same hospital admission. All participating patients signed an informed consent statement.
The major risk factors in these patients were hypertension, 63%; diabetes, 26%; hypercholesterolemia, 50%; and smoking, 64%. Associated diseases were coronary artery disease in 55%, peripheral vascular disease in 34%, pulmonary insufficiency in 8%, and renal insufficiency in 5%. Of all the patients in our series, 65% would not have satisfied the inclusion criteria for the NASCET 8,9 study; this percentage is indicative of the high-risk population treated in our study.
Study of Carotid Lesions
We routinely performed 2 studies before the procedure: one was duplex ultrasonography (Doppler echo), in order to evaluate the degree, location, and nature of the stenosis; the extent of calcification; and the presence of ulceration. Plaque echodensity was also characterized. A transcranial Doppler study completed the examination. The 2nd method of evaluation was angiography. We used digital substraction angiography with at least 2 orthogonal projections of the carotid bifurcation and performed parenchymography according to the technique of Theron and coworkers. 20 Images of the intracranial circulation were used to detect intracranial stenoses or vascular malformations that could influence treatment decisions. Most of the lesions were atheromatous (n = 284). Another 23 were restenoses, 15 of which had developed between 6 months and 13 years after endarterectomy, and 8 of which had been discovered 6 months after angioplasty. Seven lesions were related to radiation injury, and 1 was a posttraumatic aneurysm that was at high risk for cerebral embolism due to thrombi inside the aneurysm.
The arterial diameter was determined by the Quantitative Carotid Analysis method. The mean diameter of the internal carotid artery was 5.3 ± 1.2 mm. We used the NASCET angiographic criteria 8 to calculate the degree of stenosis before angioplasty and the extent of residual stenosis after stenting. The mean percentage of stenosis was 82.3% ± 8.7%; 40% of the stenoses were more than 90%. The lesions included 160 that were calcified and 166 that were ulcerated. Contralateral carotid stenosis (≥60%) was present in 53 arteries. There was thrombosis of the contralateral carotid artery in 15 cases, and associated stenosis of the ipsilateral carotid siphon—but less than that of the extracranial carotid artery—in 8 patients. The stenosis was located in the internal carotid artery in 177 cases, and the bifurcation was involved in 124 cases. Eleven patients had isolated common carotid artery stenoses, and 3 had stenoses of both the common and internal carotid arteries.
Neurologic Assessment
Neurologic examination was performed by an independent staff neurologist preprocedurally, and at 24 hours, 30 days, and 6 months after the procedure. The patients were evaluated using the N-Score for middle cerebral artery infarction devised by Orgogozo and coworkers. 21,22 A value of 100 corresponded to individuals with no neurologic deficits or to patients who had sustained a TIA. A minor stroke (deficit lasting no longer than 7 days) caused the score to be lowered by 20 points or less, and a major stroke (deficit lasting longer than 7 days) lowered the score by more than 30 points.
Procedures
Two techniques were used to treat carotid artery stenoses: the 1st was angioplasty without cerebral protection (Figs. 1 and 2). We used this technique in 165 arteries, predominantly at the beginning of our experience. Femoral access was most often used (150 cases). A 9-F guiding catheter was placed in the common carotid artery, either directly or mounted on a 0.035-inch Amplatz SuperStiff™ Guidewire (Boston Scientific Corp.; Natick, Mass) that had been placed in the external carotid artery, to maintain good support. A 3-m long, 0.018-inch coronary guide wire (Roadrunner® Extra-Support Wire Guide; Cook France s.a.r.l.; Charenton Cedex, France) was used to carefully cross the lesion. The stenosis was usually predilated with a small-diameter (3.5- or 4-mm) coronary balloon catheter to facilitate stent passage; however, this step was omitted in 75 patients. We generally used a Speedy Bypass™ catheter (Boston Scientific). A stent was delivered to the site of the lesion and dilated to a diameter equal to that of the artery. In some cases, it was not possible to selectively catheterize the carotid artery using the femoral approach due to severe arterial tortuosity. In such cases and in the presence of severe aortoiliac lesions, direct carotid artery puncture (13 cases) or a brachial approach (2 cases) was applied.
|
|
Fig. 1 Right internal and common carotid artery stenoses. |
|
|
Fig. 2 Result after deployment of 2 Palmaz® stents: 1 in the right internal carotid artery and the 2nd in the common carotid artery. |
The 2nd technique, used in 150 arteries, was angioplasty with cerebral protection (Figs. 3, 4, and 5). Two different methods of protection were used.
|
|
Fig. 3 Intracranial views showing tight stenosis of the left internal carotid artery before angioplasty. |
|
|
Fig. 4 Predilation of the left internal carotid artery lesion under cerebral protection with the PercuSurge™ device. |
|
|
Fig. 5 Final result after placement of a Palmaz® stent in the left carotid artery. |
Theron and coworkers’ technique. 20 We used this technique in 47 arteries. First, a triple coaxial catheter was used to occlude the internal carotid artery beyond the stenosis with a latex balloon. Angioplasty and stent placement were then performed. Débris generated by the procedure was aspirated through the guiding catheter placed in the internal carotid artery. Flushing of residual débris toward the external carotid artery followed the aspiration. Disadvantages, including poor radiopacity, nonsteerability, and the high profile of the catheter were noted with this technique. After observing 4 complications, we halted its routine use.
The PercuSurge® Guardwire™ temporary occlusion and aspiration system (PercuSurge, Inc.; Sunnyvale, Calif). We used this system to treat 94 lesions in 84 high-risk patients. The device consists of several components. The Guardwire is a 0.014- or 0.018-inch angioplasty wire (190 or 300 cm in length) constructed of a hollow nitinol hypotube. Incorporated in its distal segment is an inflatable elastomeric balloon capable of occluding vessel outflow. The proximal end of the wire incorporates a Microseal™, which enables inflation and deflation of the occlusion balloon using a Microseal adapter. The Guardwire is advanced through a 9-F guiding catheter previously inserted in the common carotid artery. After crossing the lesion, the occlusion balloon is placed distal to the stenosis and inflated. Angioplasty and stenting are performed. An aspiration catheter is then advanced over the wire into the vessel, and débris is retrieved by manual suction. Flushing can also be performed in order to direct residual particles into the external carotid artery.
Using this method, we achieved technical success in 93 arteries (98%). In 1 case, it was impossible to cross the lesion with the Guardwire due to severe tortuosity with calcified ulcerated stenosis of the common and internal carotid arteries. The protective balloon occlusion was well tolerated in 89 cases (95%). Two patients with poor collateral circulation developed immediate intolerance with loss of consciousness and seizures upon inflation of the balloon. In one of these patients, we deflated the balloon and continued the procedure without cerebral protection. Both patients had uneventful recoveries immediately after balloon deflation. Two other patients had transient balloon intolerance manifested by agitation and impaired verbal communication, starting about 2 minutes after balloon occlusion and resolving quickly (before the end of the procedure). Hypotension during lesion dilation was likely a factor in both patients. The mean occlusion time was 503 ± 217 seconds (range, 125 to 991 seconds).
Visible débris was removed with the aspiration catheter in all patients and analyzed. The number of particles varied from 3 to 283, with a mean of 76 particles per procedure. The mean diameter was 250 μm (range, 53 to 2,846 μm) and consisted of atheromatous plaque, necrotic core, cholesterol crystals, fibrin, organized and fresh thrombi, lipid masses, platelets, and macrophage foam cells.
With the PercuSurge technique, we observed 1 periprocedural neurologic deficit (1.1%): amaurosis fugax occurring after acute thrombosis of a Wall-stent® Endoprosthesis (Boston Scientific) treated with abciximab. This complication occurred after flushing of the residuum toward the external carotid artery in a patient who had an anastomosis between the external and internal carotid arteries.
Stents Used
Stents were deployed in all patients but 1. Most of the stents were Palmaz® stents (models P154AM and P204AM; Cordis Corporation, a Johnson & Johnson company; Warren, NJ), 199 of which were placed in 187 arteries. Nineteen stents covered the origin of the external carotid artery. The Palmaz stents were chosen as a part of our protocol, approved by the National Ethics Committee of France, for lesions located above the bifurcation.
Wallstents were implanted in 75 arteries, all covering the bifurcation and the external carotid artery. This stent was chosen primarily for lesions involving the bifurcation.
Twenty-nine nitinol self-expandable Expander™ stents (Medicorp S.A.; Villers les Nancy, France) were implanted in 26 arteries. We also deployed 10 nitinol S.M.A.R.T.™ stents (Cordis), 1 covered Jomed® Coronary Stent Graft (Jomed® France SARL, Voisins le Bretonneux, France) to treat an aneurysm, and 11 other stents.
Severe tortuosity of the internal carotid arteries was treated with stiffer guide wires and self-expandable, less rigid stents to eliminate kinking of the vessel. When balloon-expandable stents were deployed, their lengths were chosen to cover the lesion precisely, leaving adjacent plaques intact. The chosen lengths of self-expandable stents, especially Wallstents, were longer than the lesions in order to avoid faulty placement, to allow for natural variations between the common and internal carotid arteries, and to achieve full expansion into the common carotid artery.
Seventy-five stents were placed without preliminary dilation. The external carotid artery was covered by a stent in 128 cases without jeopardizing blood flow.
Medication and Monitoring of Patients
All patients received antiplatelet therapy, which comprised aspirin (250 mg/day) plus either ticlopidine (250 to 500 mg/day) or clopidogrel (75 mg/day) for at least 2 days before the procedure. During the interventional procedure, unfractionated heparin was administered intravenously as a bolus of 5,000 IU. We also administered atropine (1 mg) to reduce the risk of bradycardia due to compression of the carotid bulb during balloon inflation. Heparin administration was continued at therapeutic doses (activated clotting time ACT>200 seconds) for 1 day after the procedure. The antiplatelet therapy was continued at the same dose for 1 month. Aspirin alone (100 to 250 mg/day) was given thereafter.
Data Collection. On postprocedural day 1, all patients underwent a Doppler echo study, a computed tomographic scan, and a neurologic examination with N-Score evaluation. An angiogram was performed only if a problem was suspected. At day 30, Doppler echo and neurologic examinations were again performed. At 6 months, angiography was performed, along with the neurologic and Doppler echo examinations. The patients underwent a follow-up Doppler echo every 6 months thereafter. Angiography was repeated only if restenosis or other major problems were suspected.
Primary Clinical End Points. A primary end point was defined as 1) any postprocedural neurologic deficit that was determined to be a new or worsening transient deficit or a permanent neurologic deficit that occurred during or within 48 hours of the procedure; 2) any neurologic deficit, myocardial infarction, or incidence of death within the first 30 days after the procedure; and 3) the need for a new intervention, angioplasty, or endarterectomy at the 6-month follow-up.
Angiographic End Points. An angiographic end point was defined as a residual (>30%) stenosis after stenting, or a restenosis of more than 50% of the luminal diameter as seen on the follow-up angiogram at 6 months.
Results
Immediate Results
Balloon dilation and stent placement were technically successful in all patients but 1. In that case, it was impossible to selectively catheterize the right common carotid artery because of severe tortuosity. The mean residual stenosis after the procedure was 2.9% ± 7%. Three vertebral angioplasty procedures were performed simultaneously with balloon dilation and stent placement. Two patients with severe coronary artery disease who had undergone previous bypass surgery underwent left subclavian artery angioplasty and stenting to preserve flow to the internal mammary artery. Ultrasound examination at 24 hours showed excellent patency in all stented arteries.
Complications
There were 13 periprocedural ischemic neurologic complications (4.2%). Four patients had TIAs (1.3%), 2 of which occurred in patients older than 79 years; 4 patients had minor strokes (1.3%) with transient hemiparesis. Five major strokes (1.6%) occurred, including 3 hemiplegias. One of the patients who sustained a major stroke died 1 week later.
One patient sustained a minor stroke on postprocedural day 3 as a result of an intracerebral hemorrhage after treatment of a very tight internal carotid artery stenosis (99%); within 5 days, the condition had resolved.
Eight complications occurred after Palmaz stent deployment (4.3%) and 4 after Wallstent placement (5.4%). Five neurologic complications occurred in patients with asymptomatic lesions (2.8%) and 8 in patients with symptomatic lesions (6.3%). In all cases, the asymptomatic lesions were in patients who were at high embolic risk (advanced age, lesions with>85% stenosis, or lesions that were echolucent). Eight (4.8%) of the complications occurred in cases performed without cerebral protection, and 5 (3.6%) occurred despite the use of cerebral protection. Five of the complications, including 3 major strokes, occurred during the first 60 procedures.
Three patients developed vasospasm distal to the stenosis; this condition disappeared rapidly with vasodilator therapy. Three patients experienced local complications at the arterial entry site that required surgery: these consisted of cervical hematomas that occurred after direct puncture of the carotid artery.
No other neurologic disorder was observed during the 1st month after the procedure.
Results at 6 Months
There were 9 deaths unrelated to carotid stenting during the first 6 months after the procedure. At 6 months, 210 patients had a follow-up examination by either angiography (155) or duplex ultrasound (55). The mean percentage of angiographic stenosis was 15% ± 12% (range, 0 to 100%). We observed 1 instance of mild compression of a Palmaz stent without marked stenosis. Of 10 restenoses that were less than 50% of the luminal diameter (4.7%), 7 occurred with implantation of a Palmaz stent, 2 with a Wallstent, and 1 with a Viktor stent (Medtronic AVE; Boulogne-Billancourt, 92514, France). One case of restenosis was symptomatic: the patient presented with a TIA and was treated successfully with another angioplasty procedure. Of the 9 patients with asymptomatic restenoses, 1 had 85% restenosis with migration of the Wallstent proximally into the common carotid artery. The patient was treated successfully with a 2nd Wallstent. In 6 patients, the restenoses were treated by repeat angioplasty. Another of these patients had clinically silent stent thrombosis in the internal carotid artery. The patient was given medical treatment alone. The last case of clinically silent restenosis occurred in a patient with a Viktor stent that was impossible to redilate. This patient was treated surgically with good results.
In addition, 1 patient developed thrombosis in an external carotid artery after Palmaz stent implantation. Among the patients who underwent follow-up studies, this was the only one who had external carotid artery occlusion.
Late Results
Twelve patients died of causes unrelated to carotid angioplasty. In 185 patients who were evaluated for at least 6 months, no new neurologic event or restenosis was seen. The maximum follow-up period was 50 months (mean, 17.1 ± 8.5 months). The primary patency at 4 years was 96% and the secondary patency was 99%.
Discussion
Carotid endarterectomy is the standard treatment for carotid artery stenosis; however, substantial inherent risks and limitations remain. 13–19 On the basis of results from various national and international trials, the ad hoc Committee of the American Heart Association has proposed the following inclusion criteria for patients to undergo carotid endarterectomy. 23 First, for asymptomatic patients with stenosis of more than 60%, the periprocedural risk for stroke, death, or both, must be less than 3%. Second, for symptomatic patients with stenosis greater than 70%, the periprocedural risk for stroke, death, or both must be less than 6%. Third, for patients with recurrent ste-nosis, the perioperative risk must be less than 10%. In order for carotid angioplasty to be a therapeutic alternative to surgery, its complication rates must be similar to those of endarterectomy and it must have similar or better long-term efficacy.
Our results, like those of several recently published studies, 20,24–31 have shown that carotid angioplasty with stent placement may be performed with a complication rate that is no higher than that of surgery, despite a preponderance of high-risk patients. Our major and minor neurologic complication rates were 1.6% and 2.9%, respectively.
Diethrich’s group, 25 in a series of 110 patients (117 carotid arteries) treated with stent placement, observed 2 major and 5 minor cerebrovascular accidents (6.4%). There were also 5 TIAs (4.5%). Bergeron and associates 26 treated 32 carotid arteries with stent placement in 13 cases and reported 7 complications that occurred exclusively in lesions treated without stents. Roubin and co-authors 28 reported 1 death, 2 major strokes, and 7 minor strokes in treating 152 stenoses in carotid vessels with stents. Recently, Mathias 29 presented his data on 799 carotid lesions treated in 633 patients since 1989. Angioplasty alone was used in 32.7% and stents were placed in 67.3%. The mortality rate was 0.4% (2 patients) and complication rates were as follows: TIA, 4.4%; minor stroke, 1.6%; and major stroke, 0.9%. Wholey and coworkers 30 reported data on 114 procedures performed with Palmaz stent implantation. Complications included 2 major strokes, 2 minor strokes, and 5 TIAs. These complications appeared only in the 61 symptomatic patients (8.3% of 108 patients). In a comparative study with carotid endarterectomy, Jordan and colleagues 31 encountered 7 minor strokes (6.5%), 1 major stroke (0.9%), and 4 deaths (3.7%) in 107 patients treated with stents.
Despite these encouraging data, the risk of cerebrovascular embolism is substantial. 16,20,32,33 Particles generated by angioplasty and stenting consist of atherosclerotic débris, organized thrombi, and calcified material. In a study by Ohki and colleagues, 33 the number of particles had a positive correlation with carotid stenosis of 90% or greater and with echolucent plaques, as shown by duplex ultrasonography. Mathur and associates 34 found advanced age and the presence of long or multiple lesions to be independent predictors of strokes during carotid artery stenting. Moreover, patients with symptomatic lesions seem to be at higher risk for embolic events. In Wholey and coworkers’ study, 30 embolic complications occurred exclusively in symptomatic patients. Yadav’s group 24 found that the complication rate in their series was 10.8% in symptomatic patients versus 4% in asymptomatic patients who underwent carotid artery procedures. In our series, the complication rates were 6.3% and 2.8%, respectively. It appears that symptomatic patients have more fragile lesions, which have a tendency to release their embolic content even with minimal manipulation.
Neurologic complication rates might be reduced by the use of cerebral protection techniques. Several techniques have been proposed:
- The technique described by Theron and co-authors. 20 In their series of 259 patients, Theron’s group reported no embolization during angioplasty in the 136 patients receiving cerebral protection. In our experience, however, this technique did not limit neurologic complications.
- The PercuSurge technique. This system seems safer and easier to use than Theron’s technique. The most important advantage is the ability to cross the lesion with a coronary guide wire. We observed only 1 neurologic complication related to the technique itself.
- Other experimental devices, such as filters and umbrellas, are being developed (including one of our own), but it is too early to determine if they will provide advantages over balloon protection.
Stent implantation most likely contributes to the reduction of cerebral complications. Therefore, it is becoming routine after carotid angioplasty, independent of the results of angioplasty. Stents protect against dissection and acute thrombosis, but stents alone are insufficient to protect patients from cerebral embolism. In all published series with stents, the risk of neurological complications is at least 5%.
The choice of the stent is still an open issue. Two of the most frequently implanted stents are the Palmaz and the Wallstent. The Palmaz stent can be positioned precisely at the ostium of the internal carotid artery to avoid covering the external carotid artery. Its radial force is an important advantage in maintaining luminal integrity, particularly in calcified lesions. Several authors have encountered stent deformation, 24,28,30,35,36 although infrequently. The rigidity of the Palmaz stent may make it more difficult to implant in tortuous arteries. The Wallstent has the advantage of being self-expandable. It can be implanted at the carotid bifurcation that covers the external carotid artery, without compromising arterial flow in most cases. The flexibility of the Wallstent makes implantation easier in the presence of vessel tortuosity. The perfect stent does not yet exist, but the technology is developing rapidly.
The indications for interventional treatment of carotid artery stenosis are still much debated; however, several indications seem to have gained widespread acceptance:
- High surgical risk (patients who are elderly, patients who have severe cardiopulmonary disorders that preclude general anesthesia or prolonged surgical procedures, and those with severe renal insufficiency)
- Surgical restenosis
- Post-radiation carotid stenosis
- High bifurcation (at or near the base of the skull)
- Proximal or aorto-ostial lesions
- Distal lesions that include fibromuscular dysplasia
As percutaneous interventional techniques improve, the indications may broaden. In the presence of symptoms, all patients with stenoses of 70% or greater might eventually be considered candidates for carotid angioplasty and stenting.
In the presence of asymptomatic lesions, the indications are more challenging. Surgery is beneficial to a minority of patients, and the risks are substantial. Further, stenting has not been shown to impart long-term results comparable to those of endarterectomy. In our opinion, the criteria as delineated by the ACAS study 12 are too broad. We prefer to limit our indications as follows:
- Stenosis greater than 75% to 80%
- Rapidly progressive stenosis on 2 consecutive Doppler echo studies
- Bilateral, tight stenoses
- Echolucent stenosis
- Hemodynamically important stenosis with associated vertebral or intracranial vascular lesions, the absence of collateral flow through the circle of Willis, or a combination of these
Theron and co-authors 20 emphasized that the degree of stenosis alone is not a sufficient indication for intervention: intracerebral vascular hemodynamics should always be considered.
We have observed that 65% of our patients would have been excluded temporarily or completely from the above studies due to the severity of their carotid artery disease. Half of our patients also had severe coronary disease; yet we had no cardiac complications in the immediate follow-up period. On the other hand, 3 of our patients with pedunculated thrombi and calcified ulcerations of the internal carotid artery were refused angioplasty elsewhere and were referred to us for surgery. In those cases, we agreed with that decision, since the risk of embolism in such patients would have been very high.
In comparison with surgery, we consider the following to be clear advantages of carotid angioplasty:
- It is performed through a percutaneous access under local anesthesia, which enables strict monitoring of the patient throughout the entire procedure.
- It can be applied in high-risk patients and enables treatment of lesions that cannot be accessed surgically.
- It enables combined carotid-coronary or bilateral carotid percutaneous revascularization to be performed. (In Yadav’s study, 24 16% of patients benefited from such procedures.)
- It enables concomitant treatment of lesions of the supra-aortic vessels (performed in 5 cases in our series).
- The duration of cerebral ischemia due to dilation or inflation of the protection balloon is short: 503 ± 217 seconds at most. (Surgical clamping can last from 20 to 40 minutes, and stent placement from 2 to 3 minutes. 35)
- It renders easier the treatment of surgical restenoses, which have an operative risk higher than that of the initial endarterectomy.
- The lack of a surgical incision avoids numerous local complications, such as nerve injuries, infections, and hematomas (which occur in 2% to 12.5% of postendarterectomy cases). 1–3,8
- It involves shorter hospital stays: discharge is generally 24 to 48 hours after the procedure.
Conclusion
The combination of carotid angioplasty and stent implantation seems to be safe and efficacious. The risks do not appear to be greater than those of surgery, and the combined procedure is available to high-risk patients for whom surgery is contraindicated. However, this technique is difficult, and it should be performed only by well trained, multidisciplinary teams. Indications for this combination will likely expand as techniques are improved and new methods of cerebral protection are developed.
We anticipate that cerebral protection will soon become an essential adjunct to all cerebral angioplasty procedures in order to prevent embolism. It will broaden the indications for cerebral angioplasty and will achieve complication rates lower than those of surgery, particularly for lesions associated with high embolic risks. Indeed, considering that cerebral protection may lead to very different results, we question the advisability of initiating larger randomized studies—particularly comparative studies involving surgery—without benefit of cerebral protection.
Addendum
We have developed a new cerebral protection device (the Medicorp, Henry-Amor-Frid-Rüfenacht H.A.F.R. device; Medicorp S.A.; Villers les Nancy, France) that comprises a long hemostatic valve sheath, which is advanced into the common carotid artery; and a microcatheter with a compliant protection balloon fixed at its tip, with markers on its center for good radiologic localization. The microcatheter is mounted over a steerable 0.014-inch coronary guide wire, which is 1st placed in the internal carotid artery and maintained there during the entire procedure. This provides maximum safety for all balloon exchanges. Next, a dilation balloon is advanced over the microcatheter, the protection balloon is inflated, and the lesion is dilated. A balloon-expandable stent is then placed over the dilation balloon. If the stent is self-expandable, it is advanced directly over the microcatheter, without predilation. Débris is flushed toward the external carotid artery or aspirated.
The advantage of this technique is that any standard coronary guide wire may be used, according to the type of the lesion. The device is radiopaque, thus avoiding certain problems encountered with Theron’s technique. We have begun the 1st clinical study of this device and have treated 10 patients successfully. Thus far, this device appears to have the same advantages as the PercuSurge device.
Footnotes
Address for reprints: Michel Henry, MD, U.C.C.I. Polyclinique d’Essey, 7 rue Parmentier, 54270 Essey-les-Nancy, France
Presented at the Texas Heart® Institute’s symposium on Peripheral Interventions for the Cardiovascular Specialist, held on 4–5 November 1999, at the Marriott Medical Center Hotel, Houston, Texas
References
1. Melliere D. Carotid surgery. Assessment and current problems [in French]. J Mal Vasc 1993;18:176–85. [PubMed]
2. DeBakey ME. Carotid endarterectomy revisited. J Endovasc Surg 1996;3:4. [PubMed]
3. Zarins CK. Carotid endarterectomy: the gold standard. J Endovasc Surg 1996;3:10–5. [PubMed]
4. Roederer GO, Langlois YE, Jager KA, Primozich JF, Beach KW, Phillips DJ, et al. The natural history of carotid arterial disease in asymptomatic patients with cervical bruits. Stroke 1984;15:605–13. [PubMed]
5. Hennerici M, Hulsbomer HB, Hefter H, Lammerts D, Rautenberg W. Natural history of asymptomatic extracranial arterial disease. Results of a long-term prospective study. Brain 1987;110(Pt 3):777–91. [PubMed]
6. Whisnant JP, Wiebers DO. Clinical epidemiology of transient cerebral ischemic attacks (TIA) on the anterior and posterior circulation. In: Sundt TM Jr, editor. Occlusive cerebrovascular disease: diagnosis and surgical management. Philadelphia: WB Saunders, 1987:60–5.
7. Dennis M, Bamford J, Sandercock P, Warlow C. Prognosis of transient ischemic attacks in the Oxfordshire Community Stroke Project. Stroke 1990;21:848–53. [PubMed]
8. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1991;325:445–53. [PubMed]
9. Barnett HJ, Taylor DW, Eliasziw M, Fox AJ, Ferguson GG, Haynes RB, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1998;339:1415–25. [PubMed]
10. MRC European Carotid Surgery Trial: Interim results for symptomatic patients with severe (70–99%) or with mild (0–29%) carotid stenosis. European Carotid Surgery Trialists’ Collaborative Group. Lancet 1991;337:1235–43. [PubMed]
11. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial. Lancet 1998;351:1379–87.
12. Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA 1995;273:1421–8. [PubMed]
13. Rothwell PM, Slattery J, Warlow CP. A systematic comparison of the risks of stroke and death due to carotid endarterectomy for symptomatic and asymptomatic stenosis. Stroke 1996;27:266–9. [PubMed]
14. Sundt TM Jr, Meyer FB, Piepgras DG, Fodee NC, et al. Risk factors and operative results. In: Meyer FB, editor. Sundt’s occlusive cerebrovascular disease. 2nd ed. Philadelphia: WB Saunders, 1994:241–7.
15. Winslow CM, Solomon DH, Chassin MR, Kosecoff J, Merrick NJ, Brook RH. The appropriateness of carotid endarterectomy [published erratum appears in N Engl J Med 1988;319:124]. N Engl J Med 1988;318:721–7. [PubMed]
16. Martin JB, Gailloud P, Sugiu K, Khan H, Spadola L, Piotin M, et al. In-vitro models of human carotid atheromatous disease. In: Ninth international course book of peripheral vascular intervention. Endovascular Therapy Course; 1998 May 5–8; Paris, France. Toulouse: Europa Organisation, 1998:541–6.
17. Lusby RJ, Wylie EJ. Complications of carotid endarterectomy. Surg Clin North Am 1983;63:1293–302. [PubMed]
18. Zierler RE, Bandyk DF, Thiele BL, Strandness DE Jr. Carotid artery stenosis following endarterectomy. Arch Surg 1982;117:1408–15. [PubMed]
19. Edwards WH Jr, Edwards WH Sr, Mulherin JL Jr, Martin RS 3d. Recurrent carotid artery stenosis. Resection with autogenous vein replacement. Ann Surg 1989;209:662–9. [PMC free article] [PubMed]
20. Theron JG, Payelle GG, Coskun O, Huet HF, Guimaraens L. Carotid artery stenosis: treatment with protected balloon angioplasty and stent placement. Radiology 1996; 201:627–36. [PubMed]
21. Orgogozo JM, Capildeo R, Anagnostou CN, Juge O, Pere JJ, Dartigues JF, et al. Development of a neurological score for the clinical evaluation of Sylvian infarctions [in French]. Presse Med 1983;12:3039–44. [PubMed]
22. Orgogozo JM, Dartigues JF. Clinical trials in acute brain infarction. The question of assessment criteria. In: Battistini N, Fiorani P, Courbier R, Plum R, Fieschi C, editors. Acute brain ischemia: medical and surgical therapy. New York: Raven Press, 1986:281–9.
23. Moore WS, Barnett HJ, Beebe HG, Bernstein EF, Brener BJ, Brott T, et al. Guidelines for carotid endarterectomy. A multidisciplinary consensus statement from the ad hoc Committee, American Heart Association. Stroke 1995;26: 188–201. [PubMed]
24. Yadav JS, Roubin GS, Iyers S, Vitek J, King P, Jordan WD, et al. Elective stenting of the extracranial carotid arteries. Circulation 1997;95:376–81. [PubMed]
25. Diethrich EB, Ndiaye M, Reid DB. Stenting in the carotid artery: initial experience in 110 patients. J Endovasc Surg 1996;3:42–62. [PubMed]
26. Bergeron P, Chambran P, Bianca S, Benichou H, Massonat J. Endovascular treatment of arteries with cerebral destination: failures and limits [in French]. J Mal Vasc 1996;21 (Suppl A):123–31. [PubMed]
27. Gil-Peralta A, Mayol A, Marcos JR, Gonzalez A, Ruano J, Boza F, et al. Percutaneous transluminal angioplasty of the symptomatic atherosclerotic carotid arteries. Results, complications, and follow-up. Stroke 1996;27:2271–3. [PubMed]
28. Roubin GS, Yadav S, Iyer SS, Vitek J. Carotid stent-supported angioplasty: a neurovascular intervention to prevent stroke. Am J Cardiol 1996;78(3A):8–12. [PubMed]
29. Mathias KD. Initial and long-term results of carotid PTA and stenting: Why stent? Syllabus for the 11th Annual International Symposium on Endovascular Therapy; 1999 Jan 23–27; Miami, Fla. Miami: Miami Cardiac and Vascular Institute, 1999:229–41.
30. Wholey MH, Wholey MH, Jarmolowski CR, Eles G, Levy D, Buecthel J. Endovascular stents for carotid artery occlusive disease. J Endovasc Surg 1997;4:326–38. [PubMed]
31. Jordan WD Jr, Schroeder PT, Fisher WS, McDowell HA. A comparison of angioplasty with stenting versus endar-terectomy for the treatment of carotid artery stenosis. Ann Vasc Surg 1997;11:2–8. [PubMed]
Les plaques d’athérome se déposent préférentiellement au niveau des endroits où les artères se bifurquent et où le sang sanguin présente des turbulences dues aux ondes de pouls. Un des endroits le plus fréquemment touché par ces plaques d’athérome se situe au niveau des bifurcations carotidiennes.
Onde de pouls
Le rétrécissement lié à la plaque d’athérome va être de plus en plus prononcé. Un caillot sanguin peut se former au niveau de la sténose ou la plaque d’athérome peut se rompre libérant ainsi des petites particules qui peuvent créer des embolies au niveau des artères du cerveau. La conséquence est la survenue d’un accident vasculaire cérébral.
Embolies célébrales
Il a été prouvé que le risque d’accident vasculaire cérébral était significativement plus élevé avec un degré de sténose supérieur à 75%.
Exemple d’une sténose carotidienne : (ACI=artère carotide interne, ACE=artère carotide externe)
Sténose carotidienne
Une sténose d’une artère carotide n’est pas douloureuse. Il faut donc en faire le diagnostic et l’examen le plus adapté est l’échodoppler.
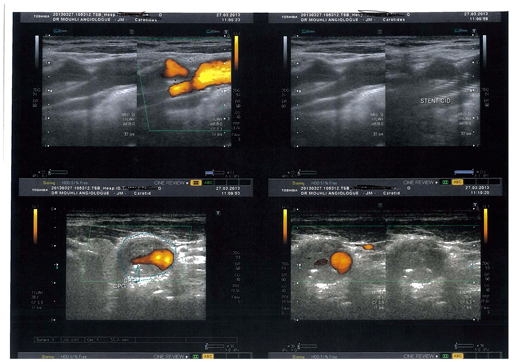
Echodoppler sténoseserrée
Les couleurs indiquent le sens et la vitesse du flux. Dans ce cas il s’agit d’une sténose de plus de 90% que l’on visualise ici au scanner.
CT sténoseserrée
Il existe deux techniques chirurgicales
1/ Endartériectomie chirurgicale
Cette opération est réalisée sous anesthésie générale ou anesthésie loco-régionale. Les artères carotidiennes sont clampées et la plaque d’athérome est enlevée puis l’artère est refermée à l’aide d’un patch veineux ou prothétique.
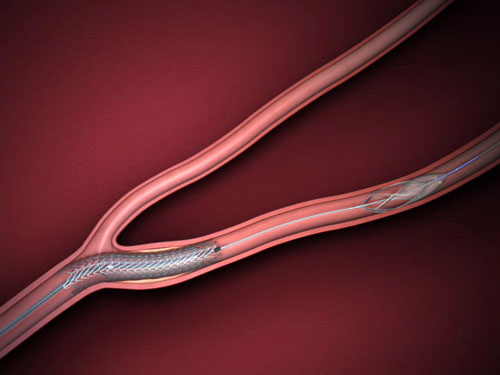
2/ Endoprothèse carotidienne
Cette procédure est réalisée sous anesthésie locale par ponction artérielle au niveau du pli inguinal. L’artère carotidienne à traiter est cathétérisée avec un guide souple. Dans la plupart des cas, on franchit la sténose avec un filtre qui permet de recueillir d’éventuels embols libérés lors de la procédure. Un stent, une prothèse métallique est ensuite mise en place au niveau de la sténose.
Les maladies cardiovasculaires constituent la première cause de mortalité dans les pays industrialisés. 44% des décès surviennent à la suite d’un accident vasculaire touchant les artères coronaires, les artères cérébrales ou d’autres parties du corps humain.


